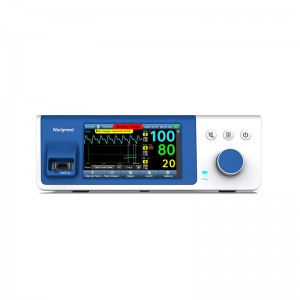
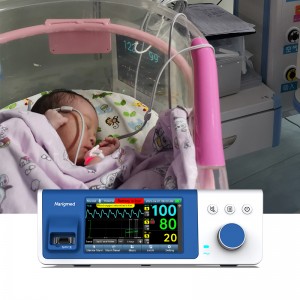
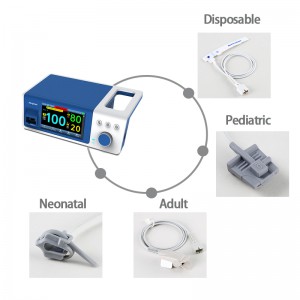
Bedside SpO2 Patient Monitoring System for neonate
Product Attributes
|
TYPE |
Bedside SpO2 Patient Monitoring System \ NICU\ICU |
|
Category |
Bedside SpO2 Patient Monitoring System for neonate |
|
Series |
narigmed® BTO-100CXX |
|
Package |
1pcs/box, 8box/carton |
|
Display type |
5.0 inch LCD |
|
Display parameter |
SPO2\PR\PI\RR |
|
SpO2 measurement range |
35%~100% |
|
SpO2 measurement Accuracy |
±2%(70%~100%) |
|
PR measurement range |
30~250bpm |
|
PR measurement Accuracy |
The greater of ±2bpm and±2% |
|
Anti-motion performance |
SpO2±3% PR ±4bpm |
|
Low perfusion performance |
SPO2 ±2%, PR ±2bpm |
|
Initial output time/Measurement time |
4s |
|
low perfusion can be supported at least |
0.025% |
|
Low perfusion performance |
SPO2 ±2%, PR ±2bpm |
|
perfusion Index Range |
0.02%~20% |
|
Respiratory rate |
4rpm~70rpm |
|
Alarm management system |
YES |
|
Probe drop detection |
YES |
|
historical trend data |
YES |
|
One click to turn off the alarm |
YES |
|
Patient type management |
YES |
|
Suitable People |
Suitable for more than 1Kg neonate OR adult |
|
Typical power consumption |
<40mA |
|
Weights |
803g(with bag ) |
|
Dismension |
26.5cm*16.8cm*9.1cm |
|
Product Status |
Self-developed products |
|
Voltage - Supply |
Type-C 5V or Lithium battery power supply |
|
Operating Temperature |
5°C ~ 40°C 15%~95%( humidity) 50kPa~107.4kPa |
|
storage environment |
-20°C ~ 55°C 15%~95%( humidity) 50kPa~107.4kPa
|
Following Features
1. Comprehensive functions: It can measure key physiological indicators such as blood oxygen saturation (Spo2), pulse rate (PR), respiratory rate (RR) and perfusion index parameters (PI) of newborns.
2. Wide heart rate range: supports measurement of an ultra-wide heart rate range and adapts to the changing characteristics of rapid heart rate fluctuations of newborns.
3. Universal use for hands and feet: Whether it is hands or feet, it can be measured accurately, solving the problem of newborns with poor peripheral circulation and weak signals.
4. Special probe and algorithm optimization: Through the specially designed probe and matching software algorithm, even in the case of poor blood circulation and insufficient perfusion in newborns, signals can be effectively captured and processed to ensure that various items can be clearly displayed. Measured value.
In summary, the Narigmed brand neonatal bedside oximeter can provide accurate and reliable monitoring of neonatal physiological parameters in clinical settings, especially for neonatal cases with unstable blood circulation or low perfusion.
1.Are you a factory?
We are the source factory of finger pulse oximeter. We have our own medical product registration certificate, production quality system certification, invention patent, etc.
We have more than ten years of technical and clinical accumulation of ICU monitors. Our products are widely used in ICU, NICU, OR, ER, etc.
We are a source factory integrating R&D, production and sales. Not only that, in the oximeter industry, we are the source of many sources. We have supplied blood oxygen modules to many well-known oximeter brand manufacturers.
(We have applied for multiple invention patents and product appearance patents related to software algorithms.)
In addition, we have a complete ISO:13485 management system, and we can assist customers with related product registration.
2. Is your blood oxygen level accurate?
Of course, accuracy is the basic requirement that we must meet for medical certification. We not only meet the basic requirements, but we even consider the accuracy in many special scenarios. For example, motion interference, weak peripheral circulation, fingers of different thicknesses, fingers of different skin colors, etc.
Our accuracy verification has more than 200 sets of comparative data covering the range of 70% to 100%, which are compared with the blood gas analysis results of human arterial blood.
The accuracy verification in the exercise state is to use exercise tooling to exercise with a certain frequency and amplitude of tapping, friction, random movement, etc., and compare the test results of the oximeter in the exercise state with the results of the blood gas analyzer for arterial blood Validation, it would be helpful for some patients such as patients with Parkinson’s disease to measure use. Such anti-exercise tests are currently only done by three American companies in the industry, masimo, nellcor, Philips, and only our family has done this verification with finger clip oximeters.
3. Why does blood oxygen fluctuate up and down?
As long as the blood oxygen fluctuates between 96% and 100%, it is within the normal range. Generally, the blood oxygen value will be relatively stable under even breathing in a quiet state. Fluctuations of one or two values in a small range are normal.
However, if the human hand has movement or other disturbances and changes in breathing, it will cause large fluctuations in blood oxygen. Therefore, we recommend that users keep quiet when measuring blood oxygen.
4. 4S fast output value, is it real value?
There are no settings such as “created value” and “fixed value” in our blood oxygen algorithm. All displayed values are based on body model collection and analysis. 4S rapid value output is based on rapid identification and processing of pulse signals captured within 4S. This requires a lot of clinical data accumulation and algorithm analysis to achieve accurate identification.
However, the premise for rapid 4S value output is that the user is still. If there is movement when the phone is turned on, the algorithm will determine the reliability of the data based on the collected waveform shape and selectively extend the measurement time.
5. Does it support OEM and customization?
We can support OEM and customization.
However, since the logo screen printing requires a separate screen printing screen and separate material and bom management, this will lead to an increase in our product cost and management cost, so we will have a minimum order quantity requirement. MOQ:1K.
The logos we can provide can appear on product packaging, manuals, and lens logos.
6. Is it possible to export?
We currently have English versions of packaging, manuals and product interfaces. And it has obtained medical certification from the European Union CE (MDR) and FDA, which can support global sales.
At the same time we also have FSC free sales certificate (China and EU)
However, for some specific countries, it is necessary to understand the local access requirements, and some countries also need a separate permit.
Which country are you exporting to? Let me confirm with the company whether that country has special regulatory requirements.
7. Is it possible to support registration in XX country?
Some countries require additional registration for agents. If an agent wants to register our products in that country, you can ask the agent to confirm what information they need from us. We can support providing the following information:
510K authorization certificate
CE (MDR) authorization certificate
ISO13485 qualification certificate
Product information
According to the situation, the following materials can be optionally provided (need to be approved by the sales manager):
General Safety Inspection Report for Medical Devices
Electromagnetic compatibility test report
Biocompatibility test report
Product clinical report
8. Do you have a medical qualification certificate?
We have done domestic medical device registration and certification, FDA’s 510K certification, CE certification (MDR), and ISO13485 certification.
Among them, we got the CE certification (CE0123) from TUV Süd (SUD), and it was certified in accordance with the new MDR regulations. Currently, we are the first domestic manufacturer of finger clip oximeter.
Regarding the production quality system, we have ISO13485 certificate and domestic production license.
In addition we have a Free Sale Certificate (FSC)
9. Is it possible to be the exclusive agent in the region?
Exclusive agency can be supported, but we need to provide you with exclusive agency rights after applying to the company for approval based on your company’s operating status and expected sales volume.
Usually it is a certain country where some big agents have great local influence and market share, and they are willing to promote our products, so they can cooperate.
10. Are your products new? How long has it been sold?
Our products are new and have been on the market for a few months. They are exclusively designed and positioned as high-end products. We currently have a small number of customers for OEM sales. Because of the registration certificate, it has not officially entered the FDA and CE markets. It will be sold in North America and the European Union after getting the registration certificate in November.
11. Have your products been sold before? What’s the review?
Although our products are new products, tens of thousands of them have been shipped so far, and the product quality is stable. We have been making oximeter for more than ten years, and we are aware of any customer feedback problems. We have done failure mode analysis (DFMEA/PFMEA) for every defect, from product design and development, production, raw material quality control, product inspection, packaging Control the quality of the whole process, such as delivery, to avoid possible risks.
In addition, our product design has its own characteristics, is very sensitive, and the client evaluation is quite high.
12. Is your product a private model? Is there any risk of infringement?
This is our private model, and we have applied for our product appearance patents and invention patents related to software algorithms.
Our company has a dedicated person responsible for the protection of intellectual property products. We have done a full analysis of intellectual property rights for our products, and at the same time made a layout for the corresponding intellectual property protection of our products and technologies.