BTO-100A Bedside SpO2 Monitoring System
Product Video
Product Features
BTO-100A Bedside SpO2 Monitoring System : Accuracy and Reliable
The BTO-100A Bedside SpO2 Monitoring System provides accurate and reliable readings even under low perfusion ad motion-induced signal interference, allowing clinicians to obtain continuous monitoring of patients' SpO2,pulse rate, blood pressure, temperature and respiratory status.

BTO-100A Bedside SpO2 Monitoring System FOR ICU/NICU
Narigmed’s
BTO-100A Bedside SpO2 Monitoring System offers reliable and accurate health monitoring across various conditions and environments, providing comprehensive care for patient.
1. Simplicity and Small Size:Asy to operate and intuitive, with a space-saving design.
2. Unigue Technology: Incorporates Narigmed's unigue Dynamic OxySinal Capture Technology.
3. Advance for Anti-motion: Provides precise measurements even under motion interference.
4. Accurate for Low Perfusion: Probides precise measurements even in low perfusion conditions PI≥0.025%.
5.User-Friendly Interface: Features a large font interface tailored for neonates and a trend graph on the main screen for easy visualization of changes.
6. Display and Alerts: Equipped with a 5-inch LCD color display for easy viewing, variable tone beep alerts for clinicians to monitor SpO2 changes, and a mute button with a countdown timer for convenient operation.
7. Data Management: Offers 96-hour trend memory, capures data every 4 seconds,and allows patient trend data export to a PC for archiving and analysis.
8. Battery-Efficient:Battery last 6-8 hour at a time.
Product Advantages
Utilizes the latest Narigmed’s Dynamic OxySignal Capture Technology
The BTO-100A Bedside SpO2Monitoring System offers exceptional performance in low perfusion conditions, ensuring accurate blood oxygen (SpO2 ±2%) and pulse rate (PR ±2bpm) measurements even when blood flow is minimal. This makes it ideal for patients with poor circulation, providing reliable readings when needed most. With rapid response time and high sensitivity, the BTO-100A Bedside SpO2Monitoring System ensures precise monitoring in challenging conditions.

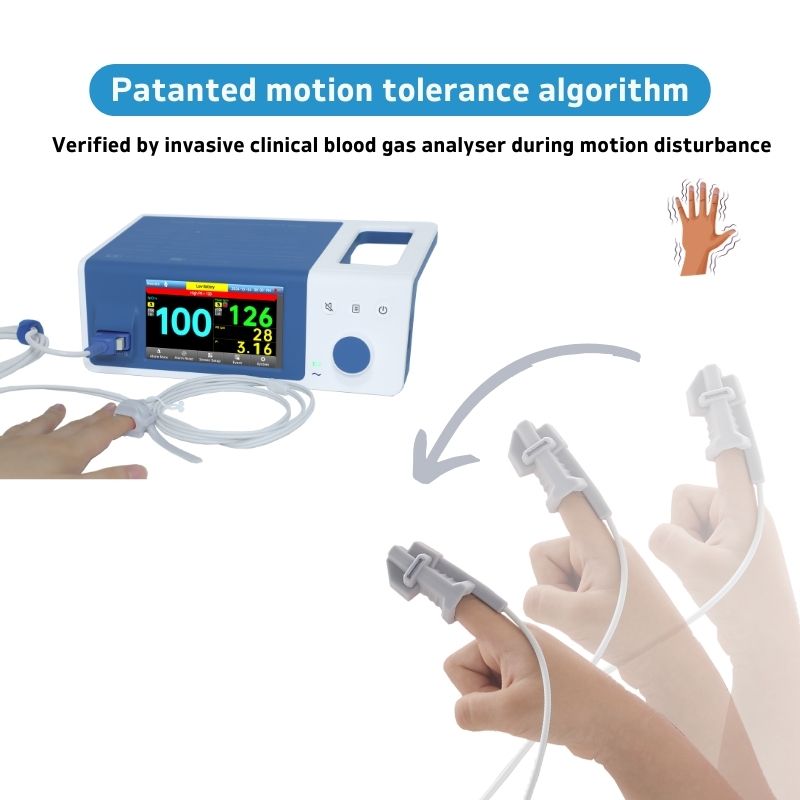
Narigmed's Unique Patented Anti-Motion Algorithm
Our oximeters are excellent in anti-motion performance, maintaining pulse rate and blood oxygen value measurement accuracy within ±4bpm and ±3% even during continuous finger shaking or interval shaking. Whether you are in the healthy population or Parkinson's patients, you can ensure reliable and accurate readings. The reliable design and advanced technology of the device make it very suitable for obtaining accurate and reliable test results of medical standards in motion.
Continuous, Reliable Monitoring,Good Partner for Oxygenerator and Ventilators
The BTO-100A Bedside SpO2 Monitoring System features high sensitivity for capturing oxygen desaturation events and supports long-term nighttime monitoring. It can be used while charging, making it ideal for continuous health tracking during sleep and ensuring comprehensive care for patients.

Rapid Measurement Within 4 Seconds Faster than competitorsp
Patented motion tolerance algorithm combined with excellentphysiological signal identification, showing results within 4 seconds.

Provides large font display interface for easy viewing
Intuitive, color user interface for ease of use and readability.

Our Services
According to customer needs, you can customize the color box LOGO, choose the charging base, customize the probe type, and customize the charging adaptation standard.


1.Are you a factory?
We are the source factory of finger pulse oximeter. We have our own medical product registration certificate, production quality system certification, invention patent, etc.
We have more than ten years of technical and clinical accumulation of ICU monitors. Our products are widely used in ICU, NICU, OR, ER, etc.
We are a source factory integrating R&D, production and sales. Not only that, in the oximeter industry, we are the source of many sources. We have supplied blood oxygen modules to many well-known oximeter brand manufacturers.
(We have applied for multiple invention patents and product appearance patents related to software algorithms.)
In addition, we have a complete ISO:13485 management system, and we can assist customers with related product registration.
2. Is your blood oxygen level accurate?
Of course, accuracy is the basic requirement that we must meet for medical certification. We not only meet the basic requirements, but we even consider the accuracy in many special scenarios. For example, motion interference, weak peripheral circulation, fingers of different thicknesses, fingers of different skin colors, etc.
Our accuracy verification has more than 200 sets of comparative data covering the range of 70% to 100%, which are compared with the blood gas analysis results of human arterial blood.
The accuracy verification in the exercise state is to use exercise tooling to exercise with a certain frequency and amplitude of tapping, friction, random movement, etc., and compare the test results of the oximeter in the exercise state with the results of the blood gas analyzer for arterial blood Validation, it would be helpful for some patients such as patients with Parkinson’s disease to measure use. Such anti-exercise tests are currently only done by three American companies in the industry, masimo, nellcor, Philips, and only our family has done this verification with finger clip oximeters.
3. Why does blood oxygen fluctuate up and down?
As long as the blood oxygen fluctuates between 96% and 100%, it is within the normal range. Generally, the blood oxygen value will be relatively stable under even breathing in a quiet state. Fluctuations of one or two values in a small range are normal.
However, if the human hand has movement or other disturbances and changes in breathing, it will cause large fluctuations in blood oxygen. Therefore, we recommend that users keep quiet when measuring blood oxygen.
4. 4S fast output value, is it real value?
There are no settings such as “created value” and “fixed value” in our blood oxygen algorithm. All displayed values are based on body model collection and analysis. 4S rapid value output is based on rapid identification and processing of pulse signals captured within 4S. This requires a lot of clinical data accumulation and algorithm analysis to achieve accurate identification.
However, the premise for rapid 4S value output is that the user is still. If there is movement when the phone is turned on, the algorithm will determine the reliability of the data based on the collected waveform shape and selectively extend the measurement time.
5. Does it support OEM and customization?
We can support OEM and customization.
However, since the logo screen printing requires a separate screen printing screen and separate material and bom management, this will lead to an increase in our product cost and management cost, so we will have a minimum order quantity requirement. MOQ:1K.
The logos we can provide can appear on product packaging, manuals, and lens logos.
6. Is it possible to export?
We currently have English versions of packaging, manuals and product interfaces. And it has obtained medical certification from the European Union CE (MDR) and FDA, which can support global sales.
At the same time we also have FSC free sales certificate (China and EU)
However, for some specific countries, it is necessary to understand the local access requirements, and some countries also need a separate permit.
Which country are you exporting to? Let me confirm with the company whether that country has special regulatory requirements.
7. Is it possible to support registration in XX country?
Some countries require additional registration for agents. If an agent wants to register our products in that country, you can ask the agent to confirm what information they need from us. We can support providing the following information:
510K authorization certificate
CE (MDR) authorization certificate
ISO13485 qualification certificate
Product information
According to the situation, the following materials can be optionally provided (need to be approved by the sales manager):
General Safety Inspection Report for Medical Devices
Electromagnetic compatibility test report
Biocompatibility test report
Product clinical report
8. Do you have a medical qualification certificate?
We have done domestic medical device registration and certification, FDA’s 510K certification, CE certification (MDR), and ISO13485 certification.
Among them, we got the CE certification (CE0123) from TUV Süd (SUD), and it was certified in accordance with the new MDR regulations. Currently, we are the first domestic manufacturer of finger clip oximeter.
Regarding the production quality system, we have ISO13485 certificate and domestic production license.
In addition we have a Free Sale Certificate (FSC)
9. Is it possible to be the exclusive agent in the region?
Exclusive agency can be supported, but we need to provide you with exclusive agency rights after applying to the company for approval based on your company’s operating status and expected sales volume.
Usually it is a certain country where some big agents have great local influence and market share, and they are willing to promote our products, so they can cooperate.
10. Are your products new? How long has it been sold?
Our products are new and have been on the market for a few months. They are exclusively designed and positioned as high-end products. We currently have a small number of customers for OEM sales. Because of the registration certificate, it has not officially entered the FDA and CE markets. It will be sold in North America and the European Union after getting the registration certificate in November.
11. Have your products been sold before? What’s the review?
Although our products are new products, tens of thousands of them have been shipped so far, and the product quality is stable. We have been making oximeter for more than ten years, and we are aware of any customer feedback problems. We have done failure mode analysis (DFMEA/PFMEA) for every defect, from product design and development, production, raw material quality control, product inspection, packaging Control the quality of the whole process, such as delivery, to avoid possible risks.
In addition, our product design has its own characteristics, is very sensitive, and the client evaluation is quite high.
12. Is your product a private model? Is there any risk of infringement?
This is our private model, and we have applied for our product appearance patents and invention patents related to software algorithms.
Our company has a dedicated person responsible for the protection of intellectual property products. We have done a full analysis of intellectual property rights for our products, and at the same time made a layout for the corresponding intellectual property protection of our products and technologies.